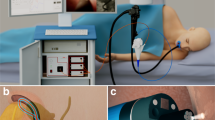
Abstract
Pancreatic cancers are usually detected at an advanced stage and have poor prognosis. About one-fifth of these arise from pancreatic cystic lesions. Yet not all lesions are precancerous, and imaging tools lack adequate accuracy for distinguishing precancerous from benign cysts. Therefore, decisions on surgical resection usually rely on endoscopic ultrasound-guided fine-needle aspiration (EUS-FNA). Unfortunately, cyst fluid often contains few cells, and fluid chemical analysis lacks accuracy—which has dire consequences, including unnecessary pancreatic surgery for benign cysts and the development of cancer. Here, we report an optical spectroscopic technique, based on a spatial gating fibre-optic probe, that predicts the malignant potential of pancreatic cystic lesions during routine diagnostic EUS-FNA procedures. In a double-blind prospective study in 25 patients, with 14 cysts measured in vivo and 13 postoperatively, the technique achieved an overall accuracy of 95%, with a 95% confidence interval of 78–99%, in cysts with definitive diagnosis.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$99.00 per year
only $8.25 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Collisson, E. A. et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat. Med. 17, 500–503 (2011).
Brand, R. E. et al. Advances in counselling and surveillance patients at risk for pancreatic cancer. Gut 56, 1460–1469 (2007).
Ryan, D. P., Hong, T. S. & Bardeesy, N. Pancreatic adenocarcinoma. N. Engl. J. Med. 371, 1039–1049 (2014).
de Oliveira, P. B., Puchnick, A., Szejnfeld, J. & Goldman, S. M. Prevalence of incidental pancreatic cysts on 3 Tesla magnetic resonance. PLoS ONE 10, e0121317 (2015).
Brugge, W. R., Lauwers, G. Y., Sahani, D., Fernandez-del Castillo, C. & Warshaw, A. L. Cystic neoplasms of the pancreas. N. Engl. J. Med. 351, 1218–1226 (2004).
Brugge, W. R. et al. Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology 126, 1330–1336 (2004).
Cizginer, S. et al. Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas 40, 1024–1028 (2011).
Distler, M., Aust, D., Weitz, J., Pilarsky, C. & Grützmann, R. Precursor lesions for sporadic pancreatic cancer: PanIN, IPMN, and MCN. Biomed. Res. Int. 2014, 474905 (2014).
Singh, M. & Maitra, A. Precursor lesions of pancreatic cancer: molecular pathology and clinical implications. Pancreatology 7, 9–19 (2007).
Birkmeyer, J. D. et al. Hospital volume and surgical mortality in the United States. N. Engl. J. Med. 346, 1128–1137 (2002).
Kosmahl, M. et al. Cystic neoplasms of the pancreas and tumor-like lesions with cystic features: a review of 418 cases and a classification proposal. Virchows. Arch. 445, 168–178 (2004).
Perelman, L. T. et al. Observation of periodic fine structure in reflectance from biological tissue: a new technique for measuring nuclear size distribution. Phys. Rev. Lett. 80, 627–630 (1998).
Qiu, L. et al. Multispectral scanning during endoscopy guides biopsy of dysplasia in Barrett’s esophagus. Nat. Med. 16, 603–606 (2010).
Sokolov, K., Drezek, R., Gossage, K. & Richards-Kortum, R. Reflectance spectroscopy with polarized light: is it sensitive to cellular and nuclear morphology. Opt. Express. 5, 302–317 (1999).
Yu, C. C. et al. Assessing epithelial cell nuclear morphology by using azimuthal light scattering spectroscopy. Opt. Lett. 31, 3119–3121 (2006).
Terry, N. G. et al. Detection of dysplasia in Barrett's esophagus with in vivo depth-resolved nuclear morphology measurements. Gastroenterology 140, 42–50 (2011).
Qiu, L. et al. Spectral imaging with scattered light: from early cancer detection to cell biology. IEEE. J. Sel. Top. Quant. Elect. 18, 1073–1083 (2012).
Wax, A. & Chalut, K. Nuclear morphology measurements with angle-resolved low coherence interferometry for application to cell biology and early cancer detection. Anal. Cell. Pathol. 34, 207–222 (2011).
Wilson, J. D. & Foster, T. H. Mie theory interpretations of light scattering from intact cells. Opt. Lett. 30, 2442–2444 (2005).
Schuele, G. et al. Optical spectroscopy noninvasively monitors response of organelles to cellular stress. J. Biomed. Opt. 10, 051404 (2005).
Itzkan, I. et al. Confocal light absorption and scattering spectroscopic microscopy monitors organelles in live cells with no exogenous labels. Proc. Natl Acad. Sci. USA 104, 17255–17260 (2007).
Mourant, J. R. et al. Light scattering from cells: the contribution of the nucleus and the effects of proliferative status. J. Biomed. Opt. 5, 131–137 (2000).
Drezek, R. et al. Light scattering from cervical cells throughout neoplastic progression: influence of nuclear morphology, DNA content, and chromatin texture. J. Biomed. Opt. 8, 7–16 (2003).
Hsiao, A., Hunter, M., Greiner, C., Gupta, S. & Georgakoudi, I. Noninvasive identification of subcellular organization and nuclear morphology features associated with leukemic cells using light-scattering spectroscopy. J. Biomed. Opt. 16, 037007 (2011).
van der Waaij, L. A., van Dullemen, H. M. & Porte, R. J. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: a pooled analysis. Gastrointest. Endosc. 62, 383–389 (2005).
Farrell, T. J., Patterson, M. S. & Wilson, B. A diffusion theory model of spatially resolved, steady-state diffuse reflectance for the noninvasive determination of tissue optical properties in vivo. Med. Phys. 19, 879–888 (1992).
Vitkin, E. et al. Photon diffusion near the point-of-entry in anisotropically scattering turbid media. Nat. Commun. 2, 587 (2011).
Chandrasekhar, S. Radiative Transfer (Dover, 1960).
Yoo, K. M., Liu, F. & Alfano, R. R. When does the diffusion approximation fail to describe photon transport in random media? Phys. Rev. Lett. 64, 2647–2650 (1990).
DeWitt, J., McGreevy, K., Schmidt, C. M. & Brugge, W. R. EUS-guided ethanol versus saline solution lavage for pancreatic cysts: a randomized, double-blind study. Gastrointest. Endosc. 70, 710–723 (2009).
Simons, J. P. et al. Malignant intraductal papillary mucinous neoplasm: are we doing the right thing? J. Surg. Res. 167, 251–257 (2011).
Oguz, D. et al. Accuracy of endoscopic ultrasound-guided fine needle aspiration cytology on the differentiation of malignant and benign pancreatic cystic lesions: a single-center experience. J. Dig. Dis. 14, 132–139 (2013).
Baker, M. S. et al. Pancreatic cystic neuroendocrine tumors: preoperative diagnosis with endoscopic ultrasound and fine-needle immunocytology. J. Gastrointest. Surg. 12, 450–456 (2008).
Winter, J. M. et al. Periampullary and pancreatic incidentaloma: a single institution's experience with an increasingly common diagnosis. Ann. Surg. 243, 673–683 (2006).
Lee, K. S., Sekhar, A., Rofsky, N. M. & Pedrosa, I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am. J. Gastroenterol. 105, 2079–2084 (2010).
Laffan, T. A. et al. Prevalence of unsuspected pancreatic cysts on MDCT. AJR Am. J. Roentgenol. 191, 802–807 (2008).
Boot, C. A review of pancreatic cyst fluid analysis in the differential diagnosis of pancreatic cyst lesions. Ann. Clin. Biochem. 51, 151–166 (2014).
Yasuda, K., Mukai, H. & Nakajima, M. Endoscopic ultrasonography diagnosis of pancreatic cancer. Gastrointest. Endosc. Clin. N. Am. 5, 699–712 (1995).
DeWitt, J. et al. Comparison of endoscopic ultrasonography and multidetector computed tomography for detecting and staging pancreatic cancer. Ann. Intern. Med. 141, 753–763 (2004).
Park, W. G. et al. Diagnostic performance of cyst fluid carcinoembryonic antigen and amylase in histologically confirmed pancreatic cysts. Pancreas 40, 42–45 (2011).
Steinberg, W. The clinical utility of the CA 19-9 tumor-associated antigen. Am. J. Gastroenterol. 85, 350–355 (1990).
Kim, J. E. et al. Clinical usefulness of carbohydrate antigen 19-9 as a screening test for pancreatic cancer in an asymptomatic population. J. Gastroenterol. Hepatol. 19, 182–186 (2004).
Khalid, A. et al. Pancreatic cyst fluid DNA analysis in evaluating pancreatic cysts: a report of the PANDA study. Gastrointest. Endosc. 69, 1095–1102 (2009).
Bick, B. L. et al. The string sign for diagnosis of mucinous pancreatic cysts. Endoscopy 47, 626–631 (2015).
Wu, J. et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci. Transl. Med. 3, 92ra66 (2011).
Amato, E. et al. Targeted next generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J. Pathol. 233, 217–227 (2014).
Wu, J. et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc. Natl Acad. Sci. USA. 108, 21188–21193 (2011).
Amelink, A., Bard, M. P., Burgers, S. A. & Sterenborg, H. J. Single-scattering spectroscopy for the endoscopic analysis of particle size in superficial layers of turbid media. Appl. Opt. 42, 4095–4101 (2003).
Mutyal, N. N. et al. In vivo risk analysis of pancreatic cancer through optical characterization of duodenal mucosa. Pancreas 44, 735–741 (2015).
Zonios, G. et al. Diffuse reflectance spectroscopy of human adenomatous colon polyps in vivo. Appl. Opt. 38, 6628–6637 (1999).
Newcombe, R. G. Two-sided confidence intervals for the single proportion: comparison of seven methods. Stat. Med. 17, 857–872 (1998).
Zhang, L. et al. Dataset for ‘Light scattering spectroscopy identifies the malignant potential of pancreatic cysts during endoscopy’. figshare http://dx.doi.org/10.6084/m9.figshare.4496039 (2017).
Acknowledgements
We thank Y. Li for help in data acquisition. This work was supported by US National Institutes of Health grants R01 EB003472 and R01 CA205431 and US National Science Foundation grants CBET-1402926 and CBET-1605116.
Author information
Authors and Affiliations
Contributions
L.Q., D.K.P. and L.T.P. conceived the method and initiated the project; L.Q. and L.T.P. supervised the project; L.Q., L.Z., U.K. and Y.Z. constructed the system; D.K.P., R.C., M.S. and T.M.B. performed clinical procedures; L.Z., Y.Z., U.K. and L.Q. performed measurements; E.U.Y., S.S. and J.D.G. evaluated the histology specimens; E.V. performed the data analysis; L.Z., E.U.Y., V.T., J.D.G., F.W., L.Q. and L.T.P. evaluated the method; S.G. performed statistical analysis; V.T., I.I., T.M.B., L.Z. and L.Q. contributed to the writing of the manuscript; L.T.P. wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary figure. (PDF 246 kb)
Supplementary Video 1
Video recording of an endoscopic ultrasound-guided fine-needle-aspiration procedure in a pancreatic cyst, combined with light scattering spectroscopic (LSS) measurements. The overall duration of the LSS data collection is less than 1.5 minutes. (AVI 16649 kb)
Supplementary Video 2
Same video recording as in Supplementary Video 1, with captions explaining the procedural steps, and with the video speed adjusted. (AVI 15042 kb)
Supplementary Video 3
Operation of the probe-latching mechanism, extending the probe tip from the needle and retracting it into the needle. The position-locking button is also shown. (AVI 7221 kb)
Rights and permissions
About this article
Cite this article
Zhang, L., Pleskow, D., Turzhitsky, V. et al. Light scattering spectroscopy identifies the malignant potential of pancreatic cysts during endoscopy. Nat Biomed Eng 1, 0040 (2017). https://doi.org/10.1038/s41551-017-0040
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41551-017-0040
This article is cited by
-
Surgical polarimetric endoscopy for the detection of laryngeal cancer
Nature Biomedical Engineering (2023)
-
In vivo detection of bile duct pre-cancer with endoscopic light scattering spectroscopy
Nature Communications (2023)
-
Estimation of porcine pancreas optical properties in the 600–1100 nm wavelength range for light-based therapies
Scientific Reports (2022)
-
Novel endoscopic optical diagnostic technologies in medical trial research: recent advancements and future prospects
BioMedical Engineering OnLine (2021)
-
A clinically translatable hyperspectral endoscopy (HySE) system for imaging the gastrointestinal tract
Nature Communications (2019)